A fuel cell is an electrochemical cell that converts energy from a fuel into electrical current and heat. Electricity is generated by the reaction between a fuel and an oxidizing agent. The reactants flow into the cell, and the reaction products flow out of it, while the electrolyte remains within it. Fuel cells can operate continuously as long as the necessary reactant and oxidant flows are maintained.
Fuel cells are different from conventional electrochemical cell batteries in that they consume reactant from an external source, which must be replenished – a thermodynamically open system.
By contrast, batteries store electrical energy chemically and hence represent a thermodynamically closed system.
Many combinations of fuels and oxidants are possible. A hydrogen fuel cell uses hydrogen as its fuel and oxygen (usually from air) as its oxidant. Other fuels include hydrocarbons and alcohols. Other oxidants include chlorine and chlorine dioxide.
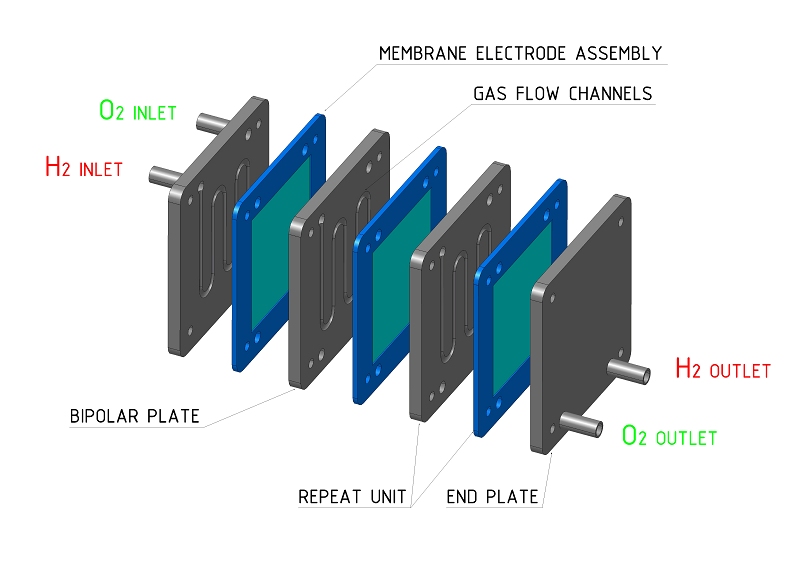
Proton exchange membrane (PEM) fuel cells operate at relatively low temperatures, offer quick start-up times and require only hydrogen fuel and oxygen from the air to operate. The core of a fuel cell system is the fuel cell stack, which is created by stacking bipolar plates (with reactant flow fields) next to membrane electrode assemblies that consist of an electrolyte (membrane) and two catalyst-coated, porous electrodes (anode and cathode). Fuel cell systems also contain a number of pumps, valves, electronics and other specialty components (balance of plant) that make up the rest of the system, including fuel supply, cooling and power management.
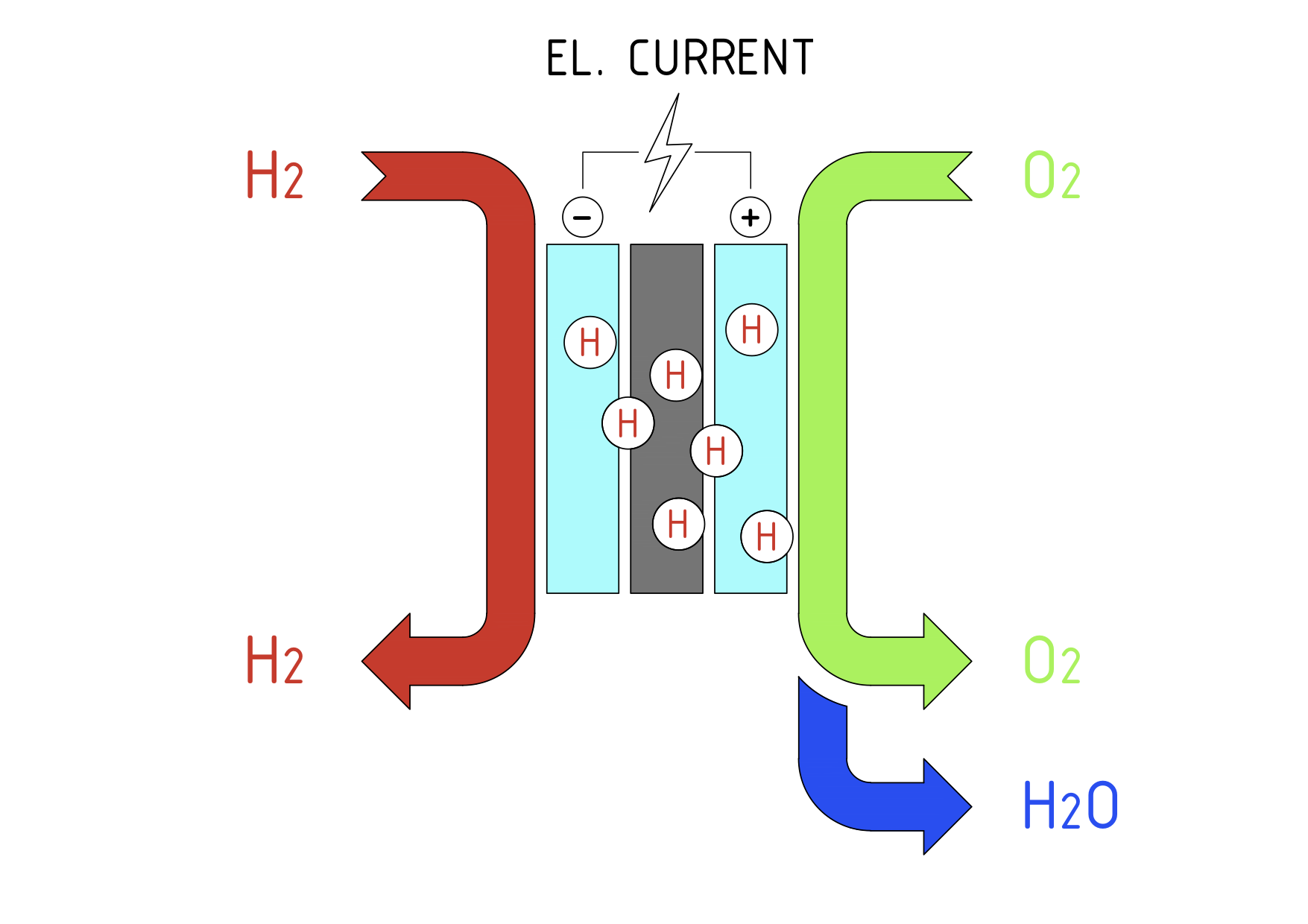
In the fuel cell system, hydrogen is fed to the anode side of the fuel cell stack where a platinum catalyst separates the hydrogen’s negatively charged electrons from positively charged ions (protons). The protons then move through the membrane to the cathode and combine with oxygen from the air and electrons, to produce water and heat. The electrons from the anode side cannot pass through the membrane and as a result are forced around the membrane through an external electrical load before returning to the cathode side of the cell. The resultant flow of electrons is a useful electrical current.
HTPEM systems are run at temperatures above 120°C, usually above 150°C and have been tested to operate normally at up to 210°C. The benefits of this elevated operating temperature are in significantly increased tolerance to impurities in fuelant gasses, such as CO presence in H2, while also benefitting the fuel cell's performance as the increase in temperature also increases the electrode kinetic rates.
A higher operating temperature also significantly reduces the need for water management, as all water in the system is in the form of vapour, thus removing 'flooding' potential, while also reducing the overall need for heat extraction from the system, resulting in fewer or smaller radiators/heat exchangers present in the system design. Since HTPEM membranes are generally not based on water, it also removes the need for MEA humidification, which further reduces system complexity and cost.
A solid oxide fuel cell is an electrochemical conversion device that produces electricity directly from fuel. In a SOFC, the electrolyte is a ceramic material, and the cells operate at temperatures from 650 to 1,000°C.
In a solid oxide fuel cell, the oxygen ions cross the electrolyte, unlike in a PEM stack where the hydrogen ions cross the membrane. This expands the list of possible fuels beyond pure hydrogen. SOFCs operate well on hydrogen and mixtures of hydrogen and carbon monoxide, among other fuels. With a very simple, small and efficient reformer integrated into the system, it is possible to produce mixtures of hydrogen and carbon monoxide from common fuels, including:
Hydrogen is the lightest and most abundant chemical element, constituting roughly 75 % of the Universe's chemical elemental mass. At standard temperature and pressure, hydrogen is a colorless, odorless, nonmetallic, tasteless, highly combustible diatomic gas with the molecular formula H2.
Industrial production is mainly from the steam reforming of methane (natural gas), and less often from more energy-intensive hydrogen production methods like the electrolysis of water. Most hydrogen is employed near its production site, with the two largest uses being fossil fuel processing (e.g., hydrocracking) and ammonia production, mostly for the fertilizer market.
Methanol is a hydrocarbon comprised of carbon, hydrogen and oxygen. Its chemical formula is CH3OH. Methanol is also known as wood alcohol because it was once produced chiefly as a byproduct of wood distillation. Today methanol is most often derived from the methane component of natural gas. It also can be produced from renewable organic materials, and the resulting organic methanol, or bio-methanol, is an alternative to petroleum-based hydrocarbons.
Methanol is a liquid at room temperature, and it is much easier to handle, package and store than hydrogen, making it a more practical fuel source. It is possible to reform the liquid methanol into hydrogen gas which is supplied directly to the fuel cell for electrical power production.